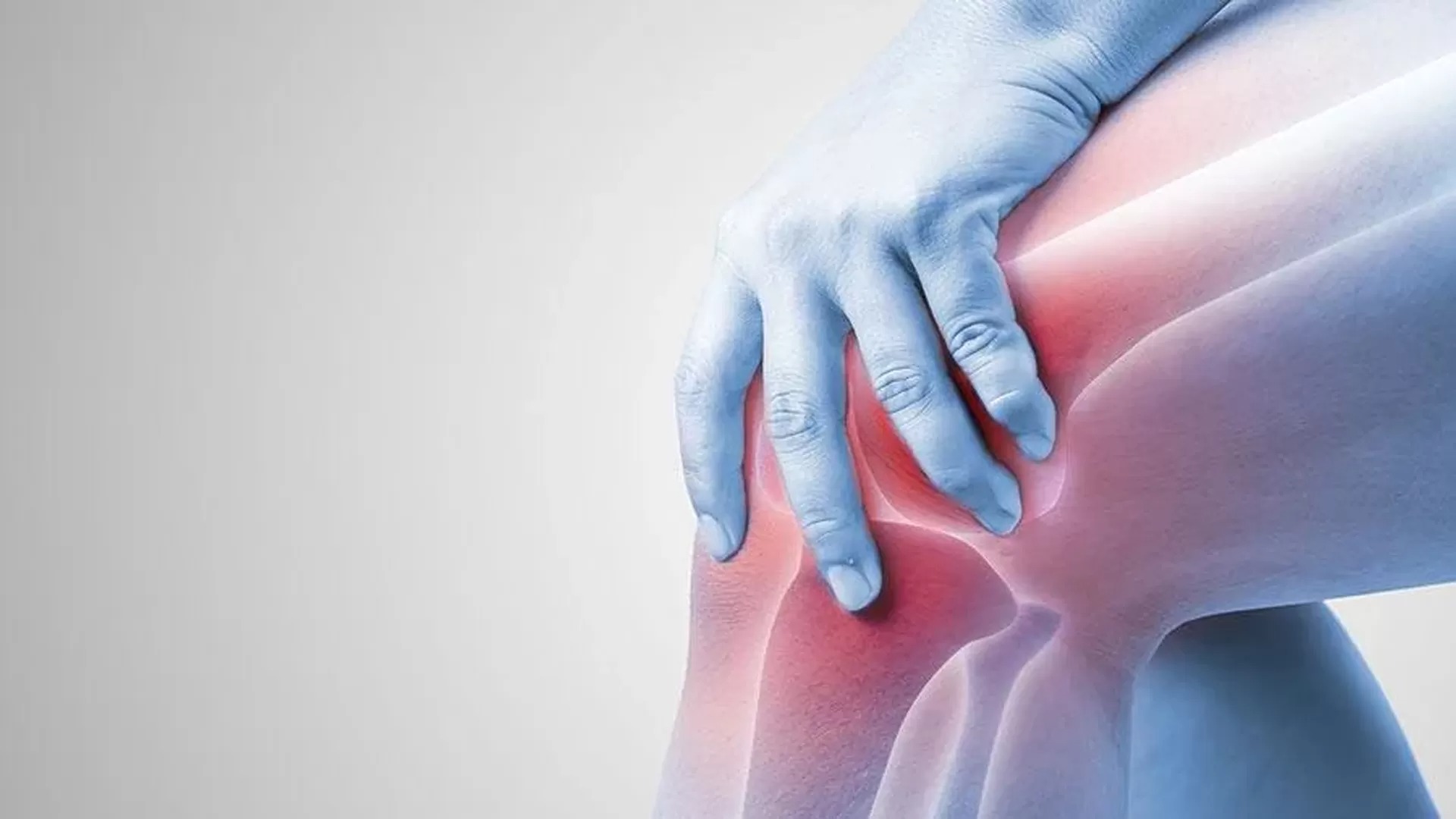
Your knees have been lying to you. All those years of accepting that cartilage damage was permanent, that arthritis was inevitable wear-and-tear, that joint replacement surgery was your eventual destiny? Stanford researchers just proved that wrong in the most dramatic way possible.
Scientists led by Nidhi Bhutani discovered that blocking a single aging enzyme can trigger remarkable cartilage regeneration—even in old, damaged joints. The breakthrough centers on 15-PGDH, an enzyme that doubles in aging cartilage and actively prevents repair by degrading healing signals. Block it with targeted injections, and something extraordinary happens.
Your Cartilage Has Hidden Regenerative Powers
The enzyme acts like a cellular bouncer, blocking prostaglandin E2 (PGE2)—your body’s natural “fix this joint” signal. As you age, 15-PGDH multiplies and shuts down repair mechanisms you didn’t even know existed. The Stanford team’s inhibitor essentially fires that bouncer, letting repair signals flow freely.
“Cartilage regeneration to such an extent in aged mice took us by surprise,” Bhutani noted. The results weren’t subtle improvements—mice showed dramatic cartilage thickening across entire joint surfaces.
Even more impressive, twice-weekly injections prevented arthritis progression in ACL injury models, the kind of knee trauma that normally carries a 50-87% arthritis risk within 5-15 years. Helen Blau, whose lab pioneered the research, emphasized the paradigm shift: “This is a new way of regenerating adult tissue… significant clinical promise for treating arthritis due to aging or injury.”
The approach doesn’t rely on stem cells or complex procedures—it simply awakens your existing cartilage cells from their aging-induced dormancy.
From Lab Bench to Your Orthopedist’s Office
Human validation came from an unexpected source: discarded knee cartilage from joint replacement surgeries. Even this end-stage tissue responded to treatment, showing reduced breakdown markers and early regeneration signs within one week. If it works on cartilage deemed beyond saving, imagine the potential for earlier intervention.
An oral version of the inhibitor already completed Phase 1 safety trials for muscle loss, establishing the compound’s human tolerability. Joint injections would localize effects while minimizing systemic exposure—think targeted repair rather than body-wide intervention.
The timeline remains realistic rather than revolutionary. Clinical trials for knee applications haven’t started, and mouse joints heal faster than human ones. But this represents the first treatment approach that addresses cartilage loss at its molecular root rather than managing symptoms after damage occurs.
Your future knee problems might become as preventable as they once seemed inevitable.